Aerogel: How it Came to Be
Aerogel: Introduction and Characteristics
Aerogel is known as the world’s lightest solid material, comprising 97% air and 3% solid structure, with a density only 1.5 times that of air. Apart from being extremely lightweight, aerogel also possesses excellent thermal insulation capabilities, mainly due to the “Knudsen effect.” As aerogel is mostly composed of silica and air, where the thermal conductivity of silica is moderate, and air has a low thermal conductivity.
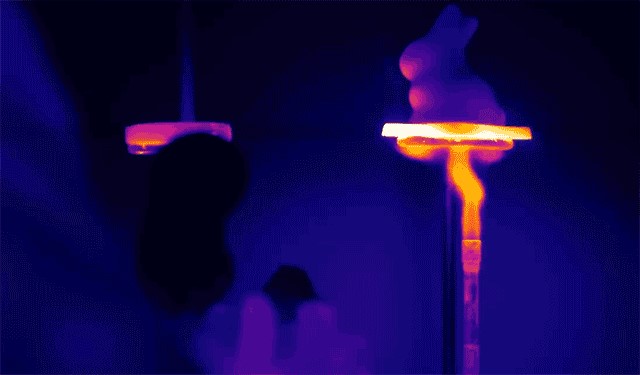
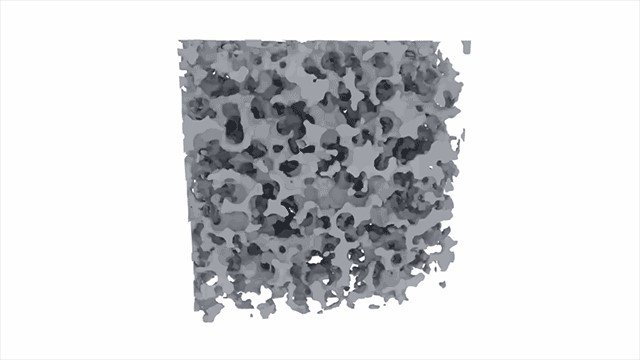
Additionally, aerogel features numerous nanoscale pores that hinder the diffusion of air through the material, impeding convective heat transfer.

Due to its high-temperature resistance, aerogel is often used in environments such as Mars rovers for insulation.
Moreover, the water-resistant property of aerogel is achieved through modification, transforming polar -OH groups into non-polar -OR, resulting in a hydrophobic aerogel.

Despite appearing as a cutting-edge product of modern technology, aerogel was first developed in the 1930s by the chemist Samuel Kistler.
The Birth of the First Aerogel
Gel-like substances are common, such as the gelatin we consume, which is a combination of solid and liquid states. Samuel Kistler and his colleague Charles Learned made a bet about the reason behind gelatin forming a gel. While Charles believed it was due to its liquid nature, Samuel argued that the key was the presence of a solid structure within the gel.
To prove his point, Samuel conducted experiments to demonstrate the presence of a continuous solid network in wet gel. The goal was to remove the liquid from the gel while retaining the solid structure, proving that gel and its liquid content were unrelated. However, the challenge was that simply allowing the liquid in the gel to evaporate would cause the solid structure to shrink due to the attractive forces between the molecules, resulting in the collapse of the gel.

To overcome this, Samuel had to replace the liquid in the gel, and the only suitable option was gas, as gel already contained both solid and liquid states. However, regular gas couldn’t replace the liquid in the gel. Samuel used a novel approach: by pressurizing and heating the gel, he made the liquid surpass its critical point, turning it into a supercritical fluid (with no distinction between liquid and gas), eliminating intermolecular attraction.
Samuel chose sodium silicate as the raw material, catalyzed by hydrochloric acid to facilitate hydrolysis. Water and ethanol served as solvents in the exchange, transforming it into an alcogel. The alcogel was then placed in a high-temperature, high-pressure environment. As ethanol reached supercritical fluid status, the gel was depressurized. With decreasing pressure, ethanol molecules were released as gas. Once removed from the heat source and cooled, the ethanol in the gel evaporated, leaving behind the solid structure filled with gas – the first aerogel.
This research was published in the journal Nature in 1931.
Improvements to the Aerogel Preparation Method

Undoubtedly groundbreaking, Samuel’s research faced stagnation for over 30 years due to challenging and time-consuming preparation conditions. It wasn’t until 1970 that the University of Lyon, seeking a porous material for storing oxygen and rocket fuel, revisited aerogel, enhancing Samuel’s method.
The new method replaced sodium silicate with tetramethoxysilane (TMOS) and ethanol with formaldehyde. This modification produced alcogels of higher quality silica aerogels and significantly reduced the time required for preparation. This improvement marked a significant step forward in aerogel science.
After these improvements, more researchers joined the field of aerogels.
In 1983, the Microstructure Materials Group at Berkeley Lab found that the highly toxic compound TMOS could be replaced by the safer tetraethoxysilane (TEOS). They also discovered that, before supercritical drying, liquid CO2 could replace the alcohol in the gel without damaging the aerogel.
This represented a major safety advancement, as CO2 lacks the explosive danger of alcohols. As research into aerogels deepened, physicists recognized that this nanoscale material could be used to collect hard-to-capture Cherenkov radiation particles, as they struggle to pass through the complex structure of the aerogel, ultimately getting trapped inside.
In addition to particle collection, silica aerogels prepared by NASA’s Jet Propulsion Laboratory were sent into space and tasked with collecting comet dust particles.

Given this comprehensive overview of aerogel’s characteristics and continuously improving preparation methods, it is clear that aerogel is an outstanding material. However, despite its merits, why hasn’t it become widely used in everyday life?
Firstly, there are challenges in production, and even though the preparation methods have been improved multiple times, the critical supercritical conditions still pose a significant barrier.
Secondly, the industrial production of aerogels faces a daunting challenge – aerogel is very brittle. While it has strong load-bearing capacity, its tensile strength is very low, making it prone to breaking with slight force. Therefore, additional additives are typically required.

